How will my Cytokine Release Assay be performed?
With our group of expert immunologists, we at iQ Biosciences are well-suited to perform cytokine release assays with your molecule to help predict potential cytokine storms in first in-human infusion trials. This page will help you understand iQ’s Cytokine Release Assay workflow.
iQ will work with you every step of the way to ensure that you are comfortable with the assay and the workflow. We start by ensuring that the isolated PBMCs and/or whole blood used in the experiment come from multiple healthy donors. Each assay will use PBMCs from, at minimum, four (4) independent donors. However, we will work with you to determine how many more independent donors you would like to meet your satisfaction and expectations.
We will then stimulate the cells with your molecule at multiple concentrations, as well as with control compounds to ensure the assay is working properly. More importantly, we will work with you and your research team to determine the best method to present your test molecule. This is relevant because the method by which the test molecule is presented has been determined to be a reason for the failure of pre-clinical studies to predict cytokine storms1,2. In addition, recent studies suggest other forms of presentation can be used to assist in prediction of infusion side effects3. Thus, we will consult with you to determine the best course of presentation for your molecule(s)/biologic(s).
Once the presentation of the test molecule is settled, and the PBMCs and/or whole blood samples are stimulated under those conditions, we will harvest the cell culture or plasma supernatant to determine the levels of multiple cytokines using cytokine bead arrays and a high-throughput flow cytometer or using Meso Scale Discovery (MSD) technology. In accordance with FDA and EMA recommendations, we generally recommend measuring IL-2, IL-6, TNF-α, and IFN-γ1,4. However, we can measure other cytokines, such as IL-4 and IL-10, that you may be interested in. In most cases, we can accommodate the measurement of any other cytokine(s) of interest using either a cytometric bead array or MSD technology for cytokine quantitation.
Finally, our research team will put together a report of the findings based on your needs. Together, we will work with you at every step of the process to ensure you are comfortable and involved. With this open communication policy, you can feel confident that your molecule is being tested properly and the results are reliable.
How will my Cytokine Release Assay readouts look?
We will present the data in an easy to understand and digestible format:
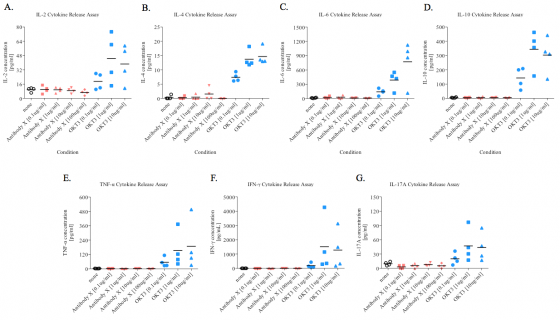
Mean serum concentration of IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and IL-17A from ‘Antibody X’ treated PBMCs.
Through the entire process, you can be assured that iQ will provide the best quality in reagents, technical skills, and service for a critical pre-clinical assay that is becoming increasingly relevant in the development of biologics.
Find out today how iQ Biosciences can help achieve your goals the smarter way.
References:
- Stebbings, R., Eastwood, D., Poole, S. & Thorpe, R. After TGN1412: recent developments in cytokine release assays. Journal of Immunotoxicology 10, 75-82 (2013).
- Eastwood, D. et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. British Journal of Pharmacology 161, 512-526 (2010).
- Scesney, S. & Ibraghimov, A. In vitro cytokine release assays for commercially available biologics for predicting cytokine storm in human patients. Immunology 2013, Honolulu, Hawaii (2013).
- Guidance for Industry Immunogenicity Assessment for Therapeutic Protein Products. FDA DRAFT GUIDANCE (Clinical/Medical, February 2013).
We are a small biotech company that critically depends on outside reagents and reliable contract research to move therapeutic candidates forward. We have turned to IQ Biosciences for access to their quality cellular reagents (including primary lymphocytes), ability to perform a wide range of in vitro assays to support our validation path, and their experience in designing and executing preclinical in vivo studies. I have been extremely pleased with their ability to execute work plans on time, communicate effectively, and apply a high level of scientific rigor that is unparalleled in my experiences with other contract organizations. Importantly, they care about the progress of our research and development.
